We help inventors navigate the rigorous steps needed to get their medical device products to the market.
We often meet people who have an idea for a medical device. They have identified a problem that they want to solve and have set about inventing a potential solution to that problem. But what then? How do you get that idea to market?
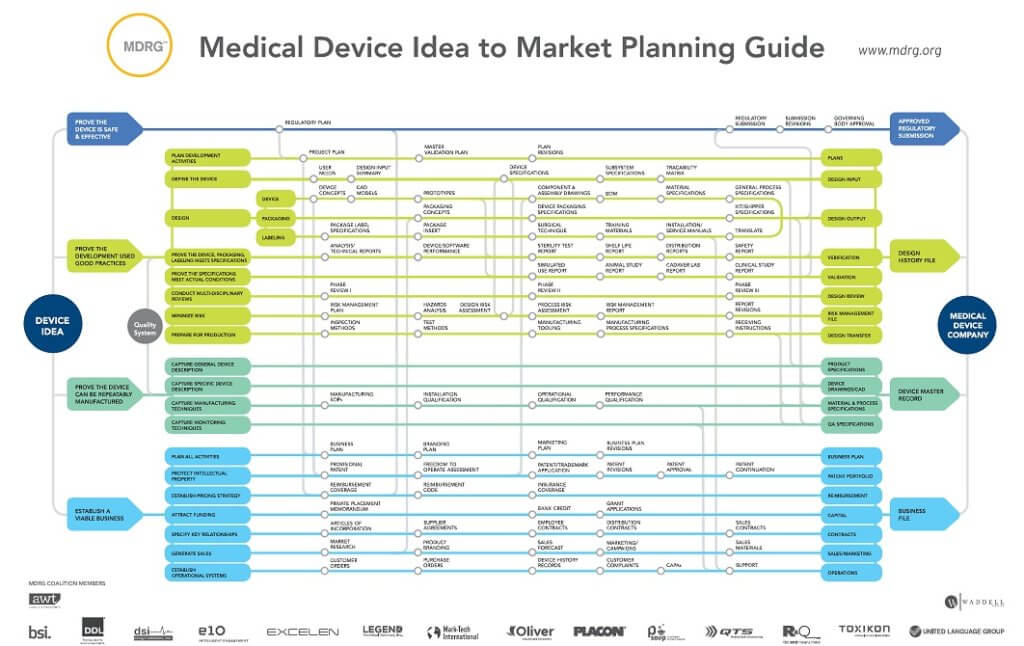
Waddell Group is a part of the Medical Device Resources Group, a consortium of businesses that help inventors get their medical device through the many steps it takes to put a device on the market for sale commercially. In order to be a resource to inventors, we have created a flow chart to help them understand the different aspects of a medical device as well as the steps required for development.
There are four categories to be aware of when creating a medical device:
- Prove the device is safe and effective
- Prove the development used good practices
- Prove the device can be repeatedly manufactured
- Establish a viable business
Prove the device is safe and effective
In the first category, you will be focusing on your regulatory plan. How are you going to prove to the Food and Drug Administration – or applicable governing body – that this device does what it is supposed to and doesn’t have harmful side effects? This is never a simple process and often you will need to engage professionals who know what the FDA is looking for and can work with you to prepare the submission documents in order for you to get an Approved Regulatory Submission.
Prove the development used good practices
The second category involves the actual creation of the device. What’s your plan? What does the device do? How are you going to design, package, and label the device? Who is going to make it? How are you going to manage your risk? This is the part where you document the design of the device and solidify your product. There are a number of roles around the creation of your device that you will likely seek professionals to assist, including design engineers, project managers, and risk managers to name just a few as you work on creating a Design History File.
Prove the device can be repeatedly manufactured
The third major category is about capturing data around the manufacture of the device. What are your processes? What are your design files? How did you choose and document the materials and techniques used in the manufacturing process? How do you make sure that you can reliably produce this product to high-quality standards? As with the former categories, you will want to identify partners that can help you document these items as you seek to create a Device Master Record.
Establish a viable business
Finally, you need to have a solid business or business unit. Just because a thing CAN be manufactured, doesn’t mean there is a viable business to go with it. What is your business plan? Who will fund the company on the front end and who will pay for the devices on the back end? Will people actually buy this product? Who is going to sell this product? How do you protect your ownership in this product? And finally, how are you going to work with your customers to make sure they are taken care of? This is essential to running any business, but the skillset that can inspire you to invent a product might not be the skillset needed to manage a business profitably. As before, all these things can be hired to help you establish your Business File.
At Waddell Group we provide World Class Project Managers to help companies navigate all these aspects of launching a product. We create project plans for the regulatory, technical, manufacturing, quality, and business elements of medical devices.
Contact the Waddell Group Today
Would you like a copy of the Medical Device Resource Group roadmap? Please email me at [email protected] to get a copy! We want to support you in your efforts to establish a viable Medical Device Company!