Many companies make this mistake when it comes to cost-effective strategies for developing medical devices. The mistake; thinking cost-effective strategies equal cost-cutting. Or at least include cost-cutting. This is not necessarily the case. Sometimes, cost-cutting is the last thing you should do when it comes to medical device development.
This article discusses some cost-effective development strategies that may seem counterintuitive. However, we have spent 20 years trying and testing these strategies in the medical device industry with great success. We can assure you they work.
Cost-Effective Strategies for Developing Medical Devices
1. Speed of development.
With medical devices, time is of the essence. Longer development cycles can result in missed market opportunities, increased expenses, and potential setbacks in achieving regulatory approvals. To ensure that your project moves efficiently(and swiftly) from concept to realization, you need to do the following.
- Instead of hiring less expensive people and possibly compromising on talent, consider hiring the best possible team from the outset. Hiring top-tier talent might seem like an expensive option at first. However, the benefits they bring are well worth the investment. They work faster, make informed decisions, and navigate challenges effectively, potentially shortening the time to market and saving costs in the long run.
- Consider developing a reimbursement strategy early in the development process. It will allow you to recoup the cost of developing and commercializing new medical devices and reduce the overall cost of medical care for patients. A variety of reimbursements are available for medical device development projects like grants, insurance, loans, tax credits, and equity financing.
- Consider the cost-quality-speed trade-off when making decisions
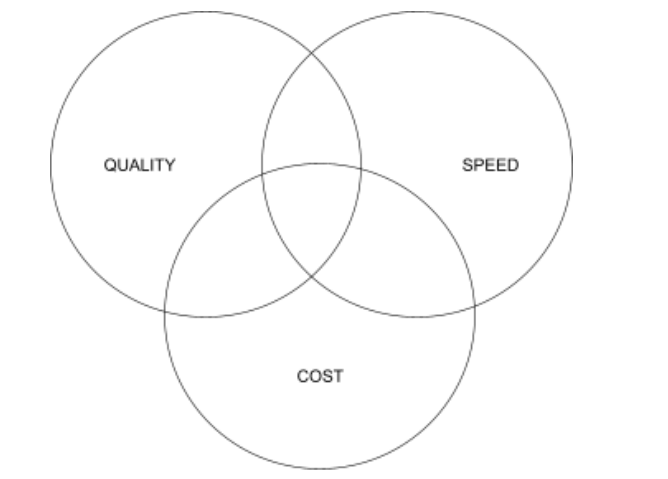
The diagram above represents the trade-off between speed, cost, and quality. It demonstrates that if you want to focus on only one element, you will have to make sacrifices for the other two as compensation. Since quality is non-negotiable in the medical device industry, there’s always a constant trade-off between speed and cost. Whether it’s making hiring decisions, allocating resources, or considering reimbursement strategies, you need to aim for a well-thought-out middle ground where quality isn’t compromised, development occurs at a brisk pace, and costs are managed diligently.
Pro tip: An example of navigating this trade-off effectively would be hiring consultants for positions not requiring full-time talent. For instance, if a part of your project demands high-end mechanical engineering, you can bring in a consultant with the required skills. Once their role is complete, you don’t incur additional costs. The per-hour cost may be higher, but you save on overheads like full-time salaries and benefits. This approach also allows you to hire top talent while maintaining flexibility and keeping your costs defined.
Looking for the right fit project management consultant to take your medical device project to market on time and budget while maintaining the quality? Get in touch!
2. Define your regulatory and quality strategies at the beginning of the project.
If your device doesn’t meet regulatory standards or quality expectations, it doesn’t reach the market. Despite this, many companies make the mistake of deferring regulatory and quality considerations until later in the process or when an issue arises. The reasons range from wanting to get to the market faster to reducing costs to lack of understanding.
This often leads to expensive mid-course corrections and delays in launching the product.
Fixing regulatory and quality problems after the fact can also be more expensive than addressing them early on in the development process. If a product is found to be unsafe or defective, it may lead to a product recall. This can damage the company’s reputation and lead to financial losses, regulatory fines, and penalties
To avoid deferring regulatory and quality considerations;
- make it a part of the company culture and
- integrate regulatory and quality considerations into the product development process from the beginning.
This will help to ensure that products are designed and manufactured to meet all applicable requirements.
3. Ensure a manufacturing engineering representative is available from the beginning of the process.
When a manufacturing engineering representative is involved in the medical device development process from day one, they can help:
- identify and resolve potential manufacturing issues early on in the development process.
- design for manufacturability (DFM) and reduce the time it takes to develop the manufacturing process.
- develop the product and the manufacturing process at the same time by working concurrently with the design team.
- reduce the overall cost of production by identifying and eliminating potential sources of scrap and rework.
- reduce the cost of warranty claims and product recalls by suggesting manufacturing processes that ensure the quality of the product.
All of this translates to a shorter development time and increased cost-effectiveness.
Simplify your product development process.
Your medical device development project will be the most cost-effective when you focus on the speed and quality of development without cutting corners for costs. If you are looking for help with that, take a peek at our product development services and reach out for a free consultation call.
First Image by redgreystock on Freepik
