
Managing any medical device project can be a challenging undertaking. From setting up your first medical device manufacturing site to complying with quality and regulatory directives to finally bringing your product to the market, the project scope is relatively large – requiring multiple communication channels across multiple functional areas.
So it makes sense when – despite the details on our services page – project managers and medical device companies ask us,
“What kind of medical device projects do Waddell Group Consultants actually work on?”
In a nutshell, Waddell Group provides medical engineering consulting and project management to medical device companies to help navigate complex regulatory and quality processes.
- Our expert medical device consultants predominantly manage projects that fall under one or more of these 8 umbrellas:
- Design and Development Projects including New product Development
- Process Development
- Building Site Location and Design
- Due Diligence for Investors and Company Boards
- Quality and Regulatory Project Management
- Recall Management
- Mergers and Acquisitions:
- Variety of Other Engagements Needing Improved Leadership and Communication
Let’s have a detailed look at what each project entails.
1. Design and Development Projects including New Product Development
The requirements to turn a medical device design into a commercially viable product differ across companies, industries, and regions. Market and regulatory requirements that hold true for European companies may not be applicable for a company in the states. A company located elsewhere but wanting to market to Europe would have different requirements. And so on. As a result, no two medical device development projects are the same.
As a medical device project management partner, our goal is to fast-track client projects to the market cost-effectively while resolving any developmental and/or regulatory issues that arise. We do that by working closely with client teams, systems, and processes while staying within the necessary geographical and industry-specific boundaries. Be it legal, quality, or regulatory.
2. Process Engineering
Process engineering in a medical device manufacturing company involves more than just optimizing a process for better yield and lower inefficiencies. It involves risk management as well as regulatory and quality compliance on top of design controls.
Waddell Group’s experienced project management consultants use a combination of lean manufacturing methodologies and the 6 sigma method to identify and implement a clear plan and process suited to an organization’s vision and needs. Some of the processes we work on are:
- Development to Manufacturing Transfer
- Project Portfolio Management
- Quality Management System Creation and Improvement
- Continuation Engineering. E.g Device Enhancements And Remediation
- Current Product Sustaining
- Assembly and Yield Improvement
- More
3. Building Site Location And Design
Building and managing a medical device manufacturing site comes with many interdependent moving parts, considerations, and challenges.
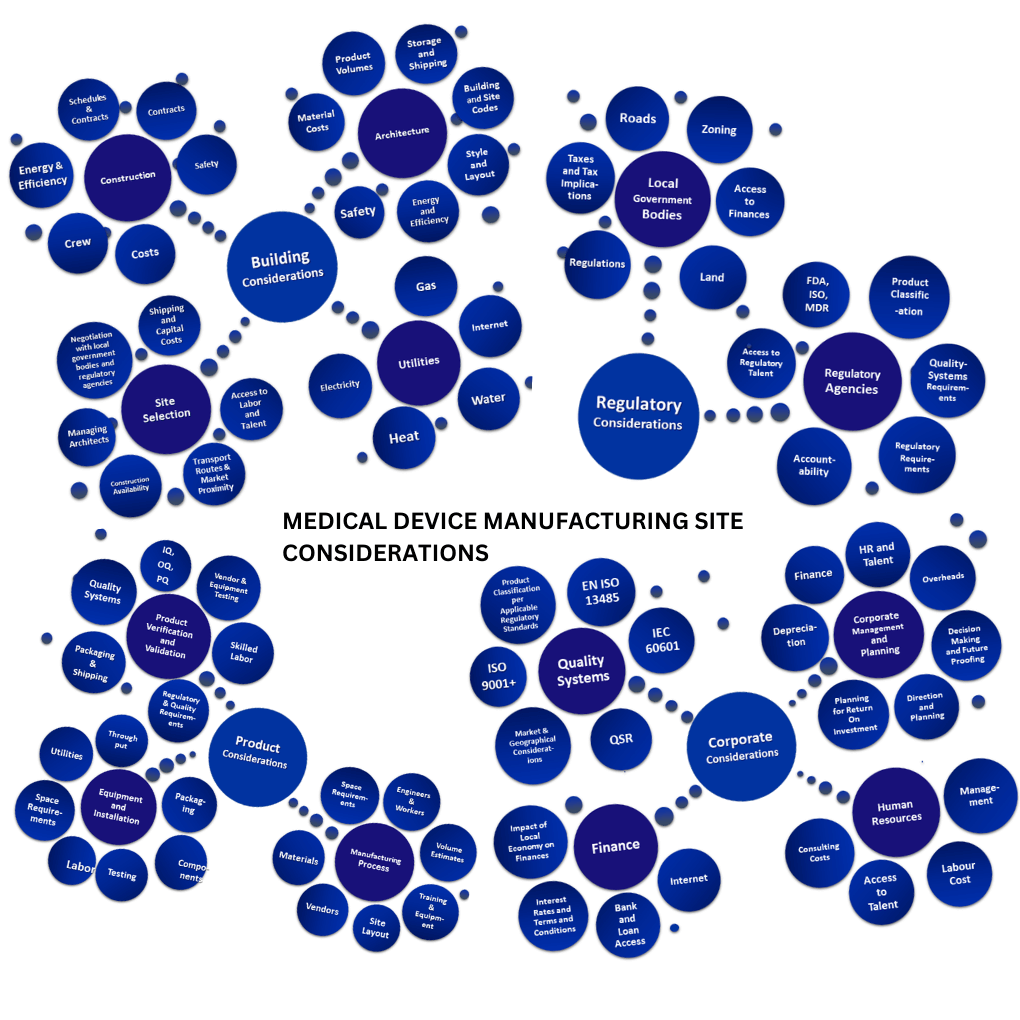
Wadell group’s expert medical device project management consultants ensure that these factors are taken care of with ease while keeping in mind, the impact and implications of any decision made on an organization’s long-term goals.
Our project management consultants and experts have assisted in building over 20 medical device manufacturing sites on time and on budget, across the globe – often with the help of verified experts and trusted partners specialized in various aspects of building new medical device manufacturing units and facilities.
4. Due Diligence For Investors And Company Boards
Due diligence for a medical device company involves more than assessing finance, litigation, and corporate structure. It also requires focused reviews of Intellectual Property, non-competes, quality management systems and certifications, and more.
At Waddell Group, our project managers perform due diligence for companies in crisis to identify what would it take to get the company back on track. We also help companies and investors looking at a company to acquire.
We help identify:
- What challenges will there be in integrating these businesses?
- What redundancies might there be with their existing business?
- How can the joined companies successfully market all their products (cross-sell, introduce new opportunities, etc.) in order to get a return on investment faster?
5. Quality and Regulatory Project Management
In order to be profitable, medical device companies need to introduce quality products to the market as soon as possible (and keep them working and marketable long-term) while staying on top of the evolving medical device regulations and quality controls. That’s where working with the right quality and regulatory project management consultants helps.
The Waddell Group guides a company’s internal team to stay on top of regulatory and quality requirements. We do that while anticipating the possible risks, and with due consideration to company finances and available resources.
6. Recall management
Most medical device companies are ill-prepared to handle an FDA 483 warning letter, much less a product recall. This risks a significant financial and reputational loss on top of low staff morale and a huge drop in market share. In order to mitigate (or avoid) these risks, it’s prudent to have someone well-versed in the best practices of recall management by your side, from the beginning.
From receiving and acting on an FDA 483 to handling warnings, compliance, consent, and recall, our team of expert consultants and project managers are well equipped to handle every aspect of medical device recall management. Including successfully reintroducing a company’s recalled flagship product back in the market.
7. Mergers and Acquisitions:
Mergers and acquisitions involve a complicated bringing together of different companies. It involves a careful review of available resources of the involved companies, the individual company cultures, their systems and processes (be it regulatory, marketing, operations, or sales), their structure and management, and more. Followed by a careful plan to assimilate and integrate all of this together into a seamless whole that creates real value for the merged company, maximizes its resources, and enhances its output, market share, and/or profit margins.
Add the highly regulated environment of the medical device industry and you have a recipe for failure unless this complexity is properly addressed.
As a part of its mergers and acquisitions management, Waddell Group offers:
- Pre-merger planning and
- Post-merger integration consulting
for medical device companies.
8. Variety Of Other Engagements Needing Improved Leadership And Communication
One of the more unique reasons we get called are projects that need senior leadership experiences, but there is an awareness that either the current team doesn’t have the talent to do the project or there will be no need for the leader once the project is completed.
The Waddell Group team has influenced change in organizations in a multitude of specializations and scenarios. Like improving departmental efficiency, turn-around of a poorly performing facility, providing strategic direction and opportunities, leadership development and so much more.
Our expert project managers and consultants can assume a full-time or fractional role to accommodate your company, and excel at successfully transitioning this role back to your internal team. We are consultants first and foremost and guarantee a smooth transition away from our leadership once the job is done.
It’s almost impossible to look at the bigger picture of the project if you are deep in the trenches focusing on one aspect of the whole. That’s where medical device project managers are an advantage.
Are You A Medical Device Company That Wants To Put Together And Manage A Highly Successful Project Team Of Your Own? Or a qualified Project Manager Looking for their next challenge?
Reach out. The Waddell group provides strategic-level project leaders for the medical device industry. Beyond essential project management skills, our highly experienced consultants know how to lead teams, manage in times of crisis, and influence change. We offer expertise other firms can’t.
